For autologous bone marrow mononuclear cells grafting in India, we utilize only bone marrow-derived cells obtained from the patient’s own body to treat conditions like Autism, Cerebral Palsy, Muscular Dystrophy, Intellectual Disability, Spinal Cord Injury, Traumatic Brain Injury, Ataxia’s, etc. Since these cells are obtained from the patient’s own body, they’re the safest, most unadulterated, and effective source of cells for autologous bone marrow mononuclear cells grafting.
Our treatment program is holistic, as we offer a unique combination of
Autologous Bone Marrow
Mononuclear Cells Grafting
NEUROREHABILITATION
We believe this multi disciplinary approach, as the key to not only treat patients with these incurable neurological conditions, but to also restore their health and vitality.
Autologous bone marrow-derived cells in India at NeuroGen BSI a minimally invasive, 100% safe and effective procedure, which requires no major surgery or incision.
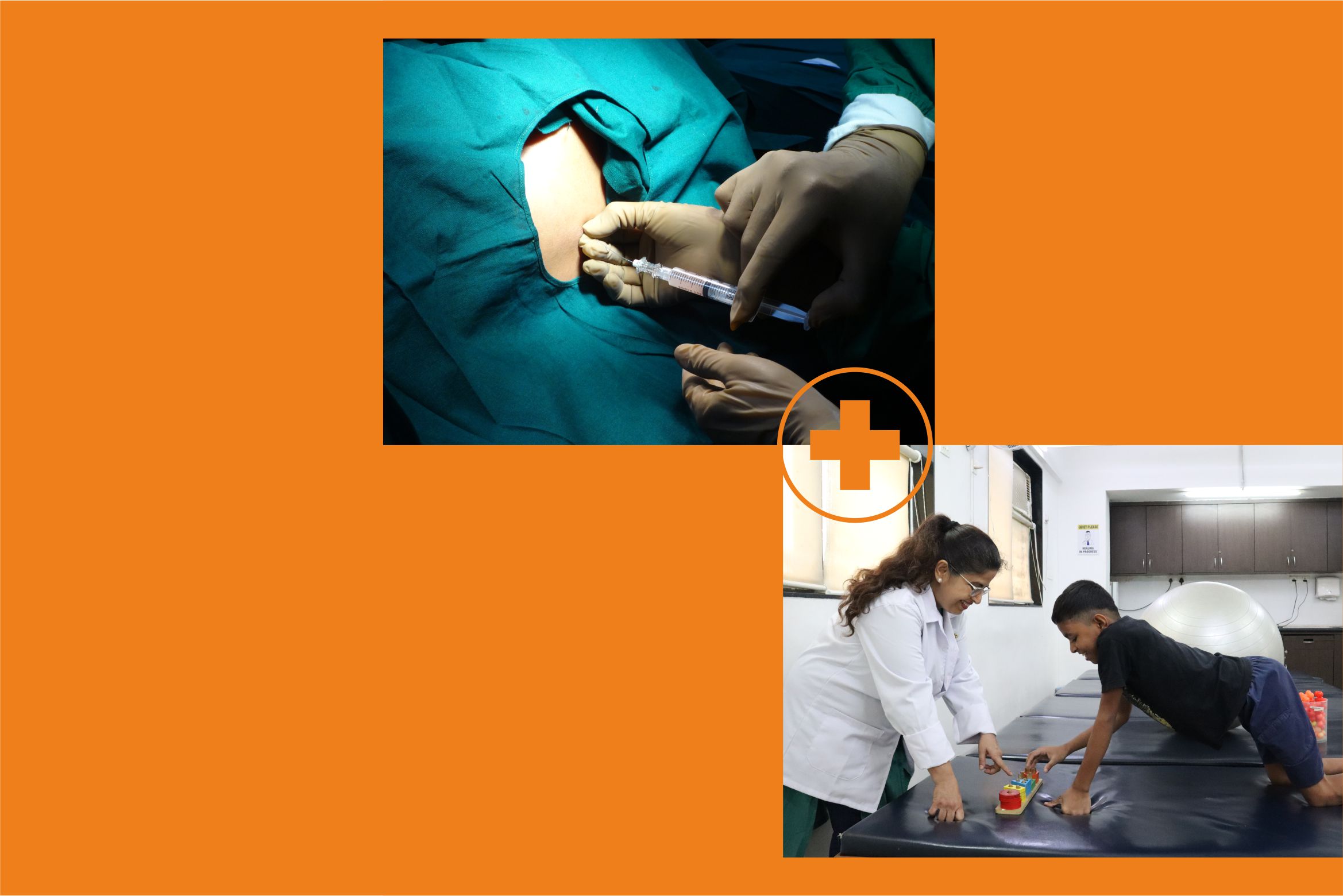
The bone marrow is a soft sponge like material found inside bones and forms blood cells. It is also a rich source of bone marrow-derived cells for autologous bone marrow mononuclear cells grafting. The hip bone is the easiest source for extraction of bone marrow-derived cells for autologous bone marrow mononuclear cells grafting. This extraction is done by a bone marrow aspiration needle, under local anaesthesia. After this, the patient is sent back to their room to rest for the next step.
The bone marrow aspirate is then transferred to the cell laboratory for processing. This is a fully equipped GLP (Good Lab Practice) & GMP (Good Medical Practice) certified laboratory, where the cells are separated from the aspirate. It takes advantage of the differences in density between the varieties of cells found in the aspirate and the density gradient medium. The separation process takes around 3 to 5 hours.
Once the BM MNCs are separated and purified (about 3-4 hours), the patient is taken back to the operation theatre. The cells are administered intrathecally (injected into the fluid around the brain and spine) using a spinal needle. In certain cases, these cells are injected into the muscles (eg. Muscular Dystrophy patients - as assessed and recommended by the rehabilitation team) using a very thin needle.
Cellular therapy at NeuroGen in India opens several scientific treatment avenues. Depending on the type of cell, there is an abundance of disorders for which autologous bone marrow mononuclear cells grafting can be considered. The ever evolving research on stem cells also builds a strong future for the untapped therapeutic potential of autologous bone marrow mononuclear cells grafting. In recent years it has been discovered that in incurable diseases and injuries, where medical science finds a dead end, autologous bone marrow mononuclear cells grafting leaps in. At NeuroGen, in India, we specialise in treating incurable neurological disorders. They are as follows :
Rapid progress in the field of biotechnology has introduced a myriad of pressing ethical issues associated with stem cell research. Worldwide and particularly in Asian countries, where autologous bone marrow mononuclear cells grafting is more advanced, the ethical basis of utilizing autologous bone marrow mononuclear cells grafting as a treatment modality is based on the Declaration of Helsinki (DoH) on the ethical principles for medical research involving human subjects. This Declaration of Helsinki is a set of ethical principles regarding human experimentation developed for the medical fraternity and is drafted by the World Medical Association (WMA). It is widely regarded as the cornerstone document on the bioethics of human research. The following is an excerpt from the Declaration of Helsinki:


